Abstract
Background
Data from clinical trials advance clinical practice, and inform treatment decisions, however trial populations may differ from real life patients. In multiple myeloma (MM), prognostic factors such as depth of response, genetic lesions, and ISS have been established largely from prospective clinical trial cohorts, and confirmation from real world data is important. Patients treated at later lines have reduced disease free and overall survival, but the importance of depth of response in relation to this remains unclear in the real world. A recent study in 7 European countries reported that depth of response correlated with longer disease free survival, however, this used physician judged disease responses1.
Aims
The aim of our study was to assess the real world outcomes of relapsed/refractory (RR) MM patients treated on clinical trials where IMWG disease responses were used, in order to confirm the clinical benefit of response depth, and other prognostic indicators. We assessed time to next treatment (TTNT) and overall survival (OS) as parameters of benefit.
Methods
Patients with RRMM treated on clinical trials at University College London (UCLH) and the National University Hospital (NUH) Singapore were identified from trial databases. Where patients were treated on more than 1 trial, the most recent was used. Patient and disease parameters were collated from clinical records and adverse risk FISH was defined as del17p, t(4;14), t1(4;16), t(14;20) or 1q gain. Survival was assessed by Kaplan-Meier methods and influence of individual factors by Cox-regression modelling on SPSS software. Survival was estimated from the day 1 of trial entry until event (date commenced next line or date of death, or date last seen if neither for TTNT and OS).
Results
Between 2009-2016, 139 patients (UCLH, 99, NUH, 40) were treated on 14 clinical trials (9 phase 3, 3 phase 2). Median age was 64years (43-78), 29 patients were >70, 87 (62.6%) were male. Median prior lines of therapy was 2 (range 1 - 6, 44.6% had 1 prior line). Of 124 patients with ISS data, 51.6% were ISS 1. Of 100 patients with FISH results, 53 had adverse risk [del17p, 23 and t(4;14), 15], 18 patients had >1 high risk lesion. A range of investigational agents was used including monoclonal antibodies, 2nd and 3rd generation proteasome inhibitors and immunomodulatory drugs, and HDAC inhibitors.
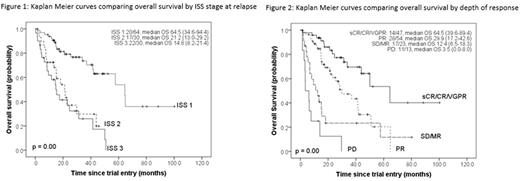
Overall response rate (ORR = CR/VGPR/PR) was 73.7%; 15 patients (10.9%) achieved sCR/CR, 32 (23.4%) VGPR and 54 (39.4%) PR. ORR did not differ between sites (UCLH 74.4%, NUH 71.1%), but was higher in patients with 1 prior line (90.3%, p=0.002), as was ³VGPR (50.0%, p=0.03). With a median follow up of 17.0 months, TTNT is 13.3months (11.2-15.5months), and OS 29.9months (15.7-43.1months); 72 patients have died.
TTNT was longer in patients with ISS 1 (p£0.01 cf ISS 2/3), and with deeper responses (sCR/CR cf PR or MR/SD, p£0.02 for both). While adverse genetics did not correlate with TTNT, t(4;14) or ≥2 adverse risk markers was associated with shorter TTNT (p£0.01 both). Increasing lines of prior therapy correlated with shorter TTNT (p<0.001).
OS was also longer in patients with ISS 1 (p<0.001 cf ISS 2/3, Figure 1), but reduced with increasing prior lines and in patients with t(4;14) (p<0.001 both). Deeper response was associated with stepwise increase in OS (sCR/CR cf SD/MR, p<0.001, Figure 2).
Multivariate models for TTNT and OS included ISS, depth of response and number of prior lines of therapy. ISS retained independent significance for both (p<0.001). There was a trend of stepwise improvement for both TTNT and OS with deeper responses (p<0.02 for both). The number of prior lines of therapy was no longer associated with TTNT or OS.
Conclusion
Our results confirm that in RRMM patients on clinical trials, depth of response is an important end-point with meaningful impact on disease free and overall survival. ISS stage at relapse continues to be a clinically useful prognostic marker, and should be included in patient assessment. The influence of genetic risk in patients treated on clinical trials may be less clear in this era of rapidly developing treatments where some adverse features may be overcome by new drug classes.
Reference:
1. Yong, K et al. (2016). Multiple myeloma: patient outcomes in real-world practice. British Journal of Haematology, 175(2), 252-264. http://doi.org/10.1111/bjh.14213
Popat: Celgene: Honoraria, Other: Travel support for meetings; Takeda: Honoraria, Other: Travel support for meetings; Amgen: Honoraria; Janssen: Honoraria, Other: Travel support for meetings. Rabin: Janssen: Consultancy, Other: Travel support for meetings, Speakers Bureau; Takeda: Consultancy, Other: Travel support for meetings, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Chng: Janssen China R&D: Research Funding. Yong: Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal